Gene Discovery, Transformation and Genomic Applications 2024
Desirable Genes/Alleles Identified in Breeding Lines
The Oklahoma State University wheat genomics team genotyped eight breeding populations using Kompetitive Allele Specific PCR (KASP) markers targeting various genes and alleles, including SBEIIa, ACCase and AHAS (Table 1). These markers, along with those for other genes of interest, were used to combine different mutations based on the breeding objectives. In total, we generated 1,645 data points from 677 individual plants or lines (Table 1). The goal was to incorporate desirable alleles into breeding lines for the development of new varieties at OSU.
| Projects | Breeding Populations | Genes | Marker | Plants/Lines | Data Points | Objectives |
|---|---|---|---|---|---|---|
| 25-1 | 25G CP7 | TaAHASL B and D | KASP | 216 plants | 273 | Wildtype, heterozygotes and mutants |
| 25-2a | 25 NID 83 | SBEII A, B and D | KASP | 22 lines | 66 | SBEII single mutants, double mutants and wildtype |
| 25-2b-1 | 24 11108 009 | SBEII A, B and D | KASP | 89 plants | 267 | SBEII triple mutants |
| 25-2b-2 | 24 11108 053 | SBEII A, B and D | KASP | 84 plants | 252 | Identification of SBEII AB double mutants |
| 25-3 | OK23D108057DF | SBEII A, B and D | KASP | 190 lines | 570 | SBEII AB double mutants |
| 25-4 | 25 101 | SBEII A, B and D | KASP | 3 plants per 5 lines | 45 | SBEII triple mutants |
| 25-5 | 25 81-82 | TaAHASL B and D | KASP | 11 lines | 22 | TaAHASL B& D single mutants, double mutants and wildtype |
| 25-6 | 25 89X | ACCase A, B and D | KASP | 50 lines | 150 | ACCase double mutants |
Genetic Signature for Phosphorus Deficiency Found in Oklahoma Wheat
Phosphorus (P) and nitrogen (N) deficiencies are major yield-limiting factors for wheat production worldwide, particularly in the acidic soils of the Southern Great Plains. In a previous study, we found the winter wheat cultivars Jagger and 2174 exhibited different coleoptile and leaf color responses to N and P deficiency. In this study, we analyzed a population of Jagger × 2174 recombinant inbred lines (RILs) and identified TaMYB-D7 on chromosome 7D – corresponding to TraesCS7D02G166500 in the Chinese Spring genome – as a major quantitative trait locus (QTL) associated with purple pigmentation in both coleoptiles and leaves. The TaMYB-D7a allele in Jagger and the TaMYB-D7b allele in 2174 differ by a single nucleotide polymorphism (SNP), resulting in an amino acid substitution: Ser-50 in TaMYB-D7a and Gly-50 in TaMYB-D7b (Figure 1).
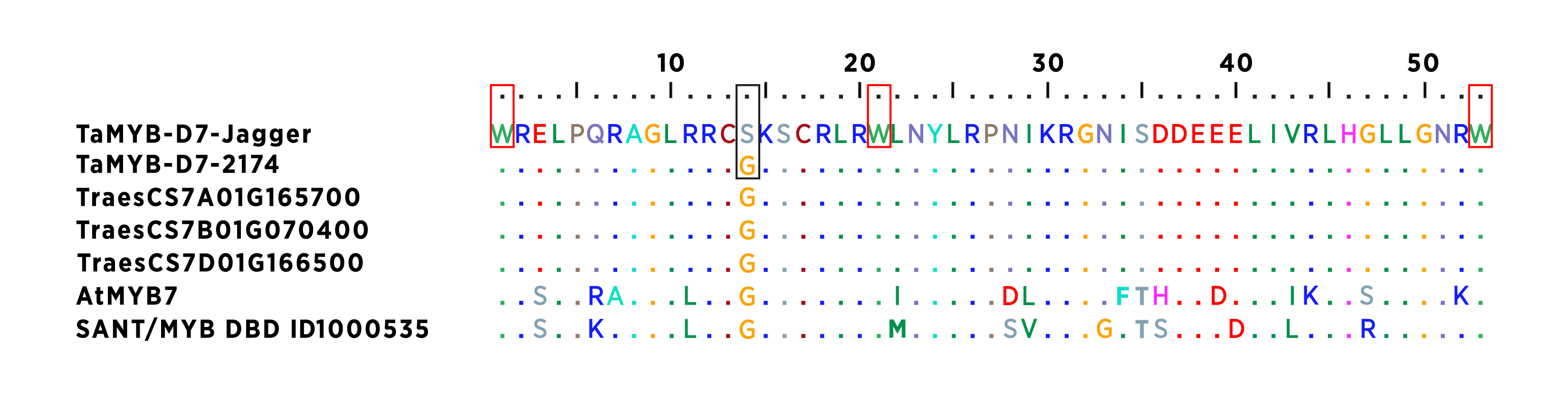
Figure1. Multiple sequence alignment of the conserved 53 amino acids in TaMYB-D7 and its homoeologs and orthologs in plants.
Three tryptophan (Trp, W) residues in the HTH sequence are highlighted with red squares. The Ser-50-Gly substitution between TaMYB-B7a and TaMYB-D7b is highlighted with a black square.
We used genome editing to inactivate all three TaMYB7 homoeologs in 2174 to validate their functions. Two single guide RNAs (sgRNAs) were designed to target TaMYB7 using the CRISPR/Cas9 system. The first sgRNA was located 9 bp downstream of the ATG start codon, while the second was positioned 50 bp downstream. Both sgRNAs targeted exon 1, with a 19 bp separation between the protospacer adjacent motif (PAM) of sgRNA1 and the start of sgRNA2 (Figure 2).
The CRISPR/Cas9 gene-editing construct containing the two sgRNAs was transformed into 2174. We identified one T₀ positive plant. Using this plant, we generated a T₁ progeny and detected several editing events across the three homoeologous TaMYB7 genes. The edited sequences were successfully genotyped using PCR markers developed in this study (Figure 2).
Figure 2a-l. Editing events at TaMYB7. The two sgRNAs are shown in the gray rectangles, and the protospacer adjacent motifs (PAMs) are denoted by boldface type. The ATG (translation start site) is shown in red, and the sequence between the ATG and the first sgRNA is shown in blue.
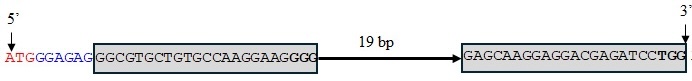
Figure 2a. Wild-type sequence of TaMYB-D7b.
![]()
Figure 2b. Sequencing of the TaMYB-D7b-ED1 editing event. Deleted bases are shown as red hyphens.
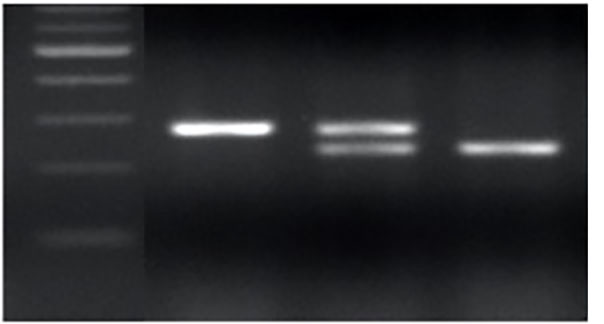
Figure 2c. PCR genotyping for the deletion in TaMYB-D7b-ED1.

Figure 2d. Wild-type sequence of TaMYB-A7.

Figure 2e. Sequence of the TaMYB-A7-ED1 editing event, showing a 149-bp deletion upstream of the first sgRNA.

Figure 2f. Sequence of the TaMYB-A7-ED2 editing event.
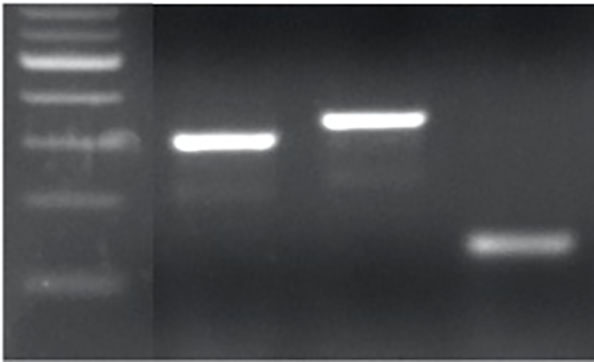
Figure 2g. PCR genotyping for the deletions in TaMYB-D7b-ED1 and TaMYB-A7-ED2.
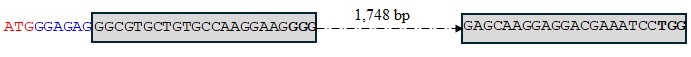
Figure 2h. Wild-type sequence of TaMYB-B7.

Figure 2i. Sequence of the TaMYB-B7-ED1 editing event.
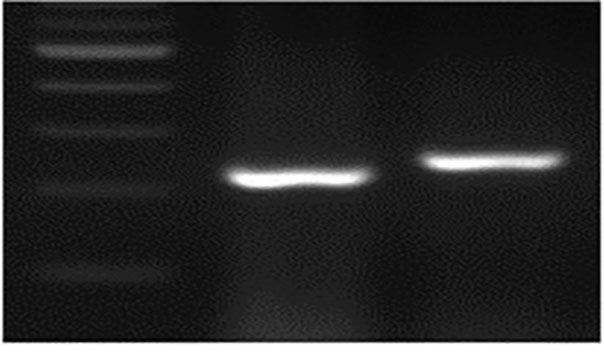
Figure 2j. PCR genotyping for the deletion in TaMYB-B7-ED1.
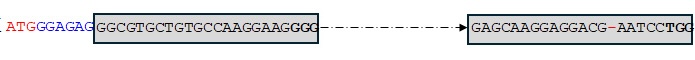
Figure 2k. Sequence of the TaMYB-B7-ED2 editing event.
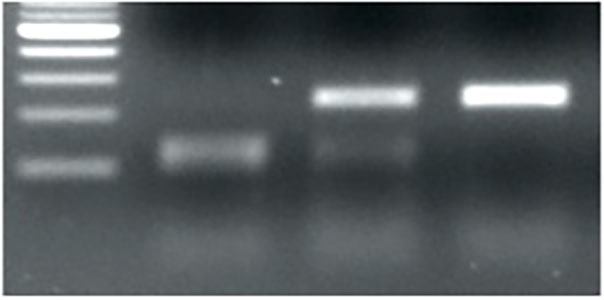
Figure 2l. PCR genotyping for the 1-bp deletion in TaMYB-B7-ED2. M is for DNA marker; H is for heterozygous.
The resulting edited wheat plants failed to accumulate purple pigmentation throughout their life cycle, confirming that TaMYB-7Db is responsible for the purple phenotype (Figure 3A & 3B). Additionally, in TaMYB7-edited plants, chalcone synthase 2-like (TaCHSL2), a gene potentially involved in anthocyanidin biosynthesis and metabolism, was significantly downregulated, suggesting TaMYB7 regulates its transcription.
Figure 3a-d. Effects of edited TaMYB7 genes in wheat.
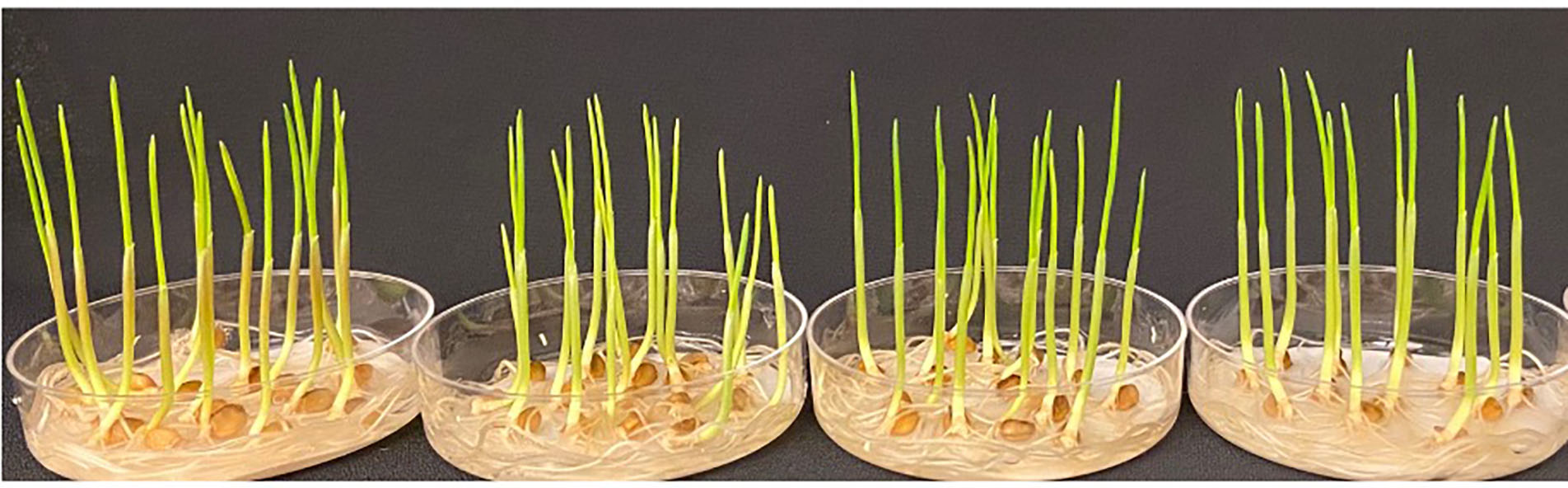
Figure 3a. WT, a2a2b2b2d1d1, a1a1b2b2DD and a2a2BBd1d1. Representative photographs of 2174 seedings (WT) and 2174 seedlings edited for TaMYB7. The seeds were germinated on petri dishes with water and without any nutrients.

Figure 3b. WT, a2a2b2b2d1d1, a1a1b2b2DD and a2a2BBd1d1. Representative photographs of 2174 plants (WT) and 2174 plants edited for TaMYB7. The plants were tested with commercial potting mix without adding any N or P. WT is for wild-type 2174; aa, bb, or dd represent the subgenomes with edited TaMYB7 genes in 2174.
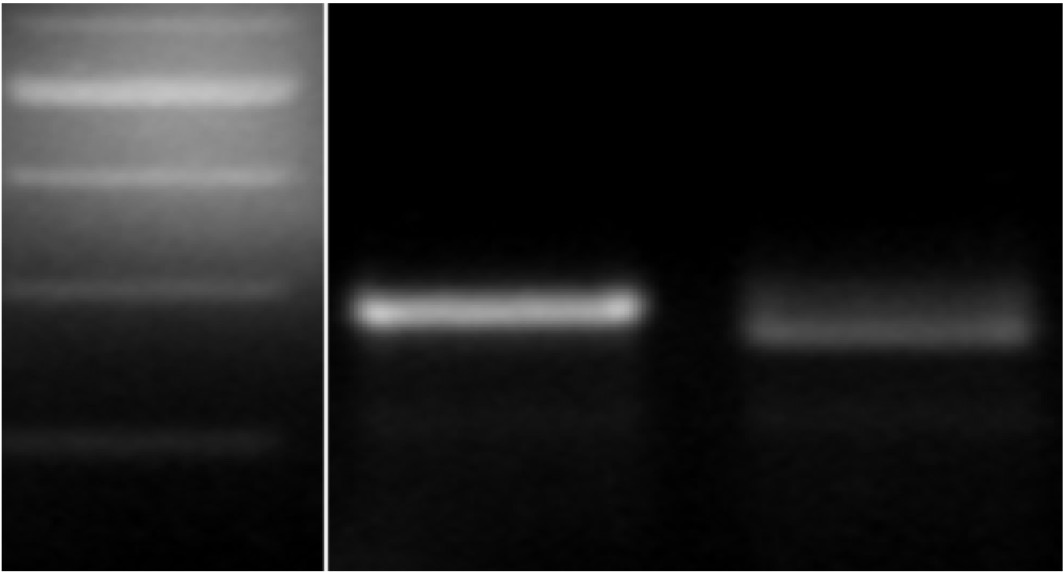
Figure 3c. 300 bp and 200 bp, M 2174 and Jagger. PCR marker for the genotyping of the TaMYB-D7 allele in Jagger and 2174. The expected size of the products after digestion with PstI is 273 bp for the 2174 TaMYB-D7b allele and 254 bp + 19 bp (out of the agarose gel) for the Jagger TaMYB-D7a allele.
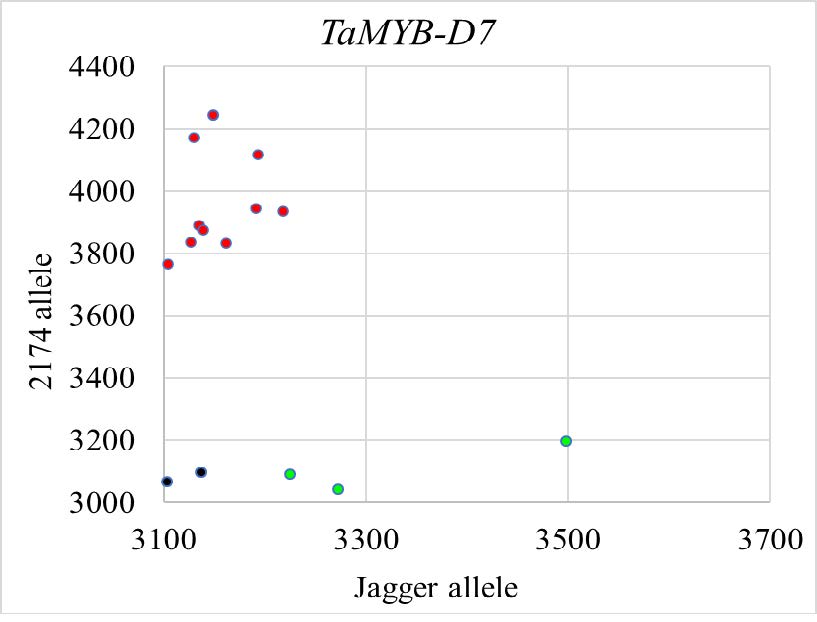
Figure 3d. KASP marker for the genotyping of the allele at TaMYB-D7. KASP-HEX for the 2174 TaMYB-D7b allele is indicated in red; KASP-FAM for the Jagger TaMYB-D7a allele in green; and the no-template control in blue.
The TaMYB-D7 gene maps closely to the previously reported Rc-D1, TaC1-D1 and TaMYB-D1 genes, which are associated with purple pigmentation in wheat coleoptiles. However, our findings reveal that the TaMYB-D7b allele also drives purple pigment accumulation in leaves under N and P deficiency. For wheat cultivars carrying the TaMYB-D7b allele, purple coleoptiles and leaves serve as visible markers of nutritional deficiency, enabling farmers to take early action to mitigate potential yield losses. The presence of purple coleoptiles is a reliable diagnostic indicator of the TaMYB-D7b allele, while purple leaves signal P and N deficiency in affected plants. In contrast, wheat cultivars like Jagger, which harbor the non-functional TaMYB-D7a allele, lack this visible phenotype. Therefore, confirming the presence of the TaMYB-D7b allele in a wheat cultivar is essential before using the absence of purple pigmentation as an indicator of nutrient sufficiency or high nutrient use efficiency.
To facilitate breeding and genetic studies, we developed a simple PCR marker to distinguish between the TaMYB-D7a and TaMYB-D7b alleles as a diagnostic and prognostic tool (Figure 3C). Additionally, we created a KASP marker for TaMYB-D7b (Figure 3D), enabling high-throughput genotyping for wheat breeding programs.
Regulation of Developmental Process by TaE3V-B1 Mediated Ubiquitination of TaVRN1 Proteins in Wheat
TaVRN1 is a central regulator of developmental phases in wheat as validated by extensive studies. In a previous study, we found that TaVRN-A1 controls the differential vernalization requirements between semi-winter wheat cultivars like Jagger, which requires three weeks of vernalization, and strong winter wheat cultivars like 2174, which require six weeks to meet their full cold exposure requirements. This variation in vernalization duration served as the basis for the positional cloning of TaVRN-A1 in winter wheat cultivars in Oklahoma. We identified a single amino acid difference between the two cultivars: Ala-180 in TaVRN-A1a (Jagger) and Val-180 in TaVRN-A1b (2174). However, the impact of this A180V substitution on the duration of vernalization in winter wheat remained unclear.
In this study, we discovered TaE3V1, a C3H2C3 RING-type E3 ligase that interacts with the TaVRN1 protein and is associated with early developmental processes. We identified two alleles, TaE3V-B1a in Jagger and TaE3V-B1b in 2174. A PCR marker was developed based on a 2-bp InDel variation between the two alleles (Figure 4A). The TaE3V-B1b allele is functional, while TaE3V-B1a possesses a 2-bp deletion that results in the loss of 43 residues (from residue 157 to residue 199) at the C terminus of TaE3V-B1a (Figure 4B).
Figure 4a-i. Natural and edited alleles of TaE3V-B1.
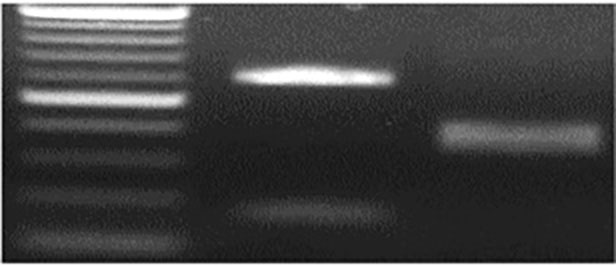
Figure 4a. M Jagger 2174. PCR marker for genotyping of TaE3V-B1 alleles. The digested PCR products were resolved on a 2% (w/v) agarose gel. M is for DNA ladder.
![]()
Figure 4b-a. 2174 TaE3V-B1b. Diagram showing the differences between TaE3V-B1a and TaE3V-B1b. The first Cys (C) highlighted in red in the C3H2C3 domain 2174 is changed to Arg (R) in the Jagger cultivar. The loss of 43 amino acids TaE3V-B1a is due to a 2-bp deletion in the Jagger genomic sequence is shown.
![]()
Figure 4b-b. Jagger TaE3V-B1a. Diagram showing the differences between TaE3V-B1a and TaE3V-B1b. The first Cys (C) highlighted in red in the C3H2C3 domain 2174 is changed to Arg (R) in the Jagger cultivar. The loss of 43 amino acids TaE3V-B1a is due to a 2-bp deletion in the Jagger genomic sequence is shown.
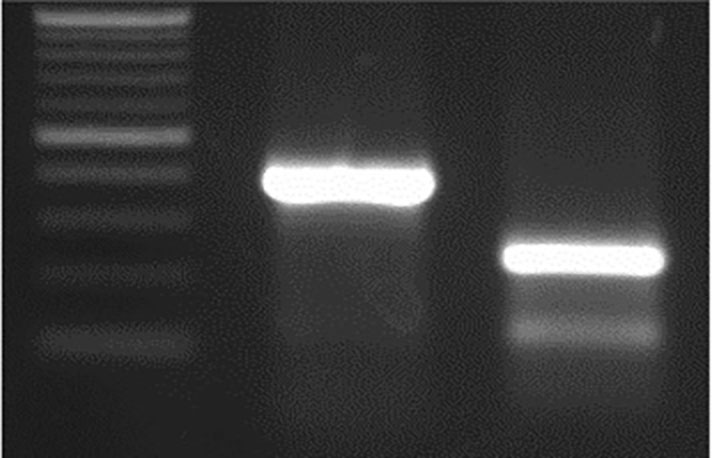
Figure 4c. M 159 bp In WT. Editing of TaE3V1 genes in transgenic plants.
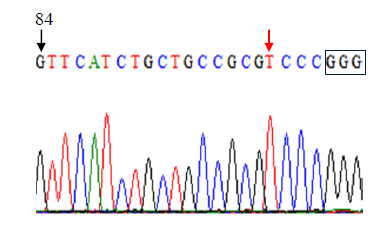
Figure 4d. Editing of TaE3V1 genes in transgenic plants. DNA sequence graph one.
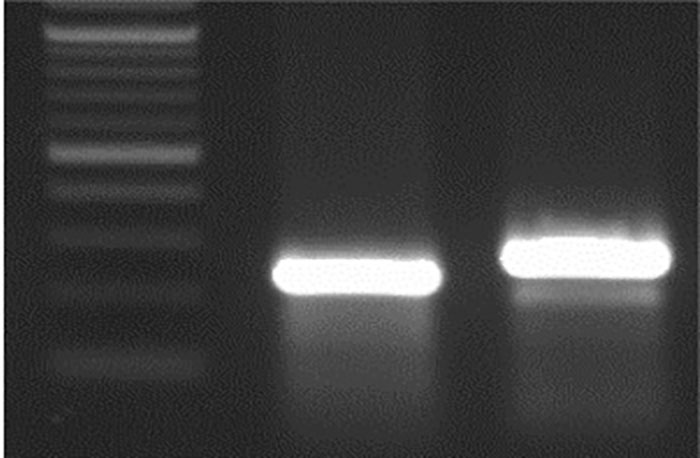
Figure 4e. M 32 bp Del WT. Editing of TaE3V1 genes in transgenic plants.
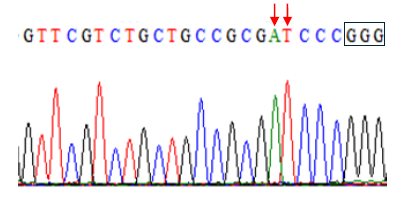
Figure 4f. Editing of TaE3V1 genes in transgenic plants. DNA sequence graph two.
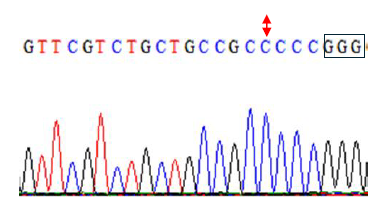
Figure 4g. Editing of TaE3V1 genes in transgenic plants. DNA sequence graph three.
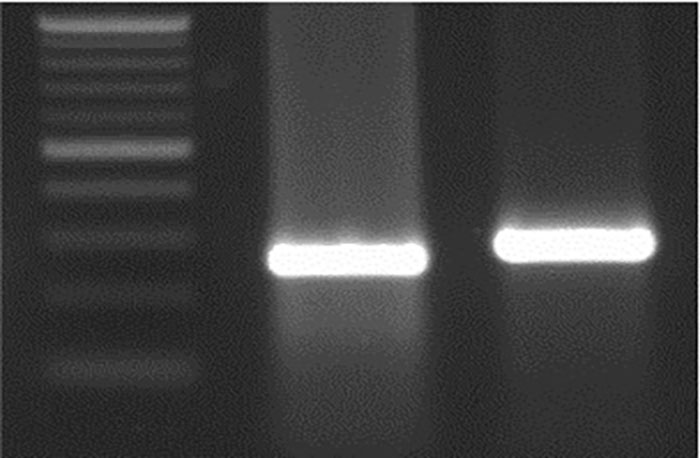
Figure 4h. M 26 bp Del WT.
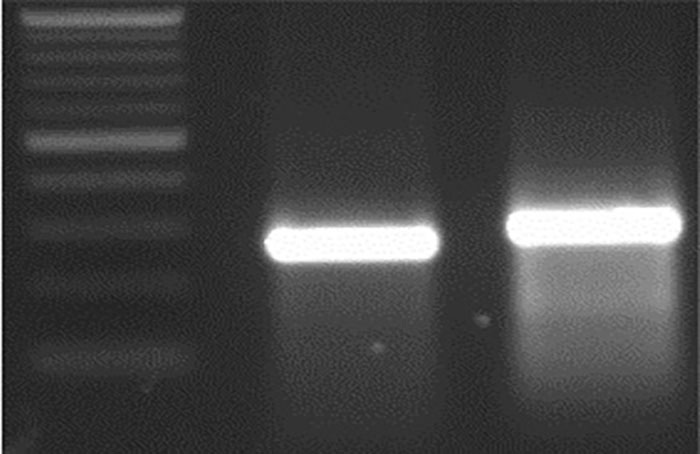
Figure 4i. M 21 bp Del WT.
We found that TaE3V-B1b, encoded by the functional allele in 2174, interacted with and ubiquitinated the TaVRN1 protein. In contrast, TaE3V-B1a, encoded by a natural non-functional allele in Jagger, neither interacted with TaVRN1 nor exhibited ubiquitination activity. Without vernalization, plants carrying the TaE3V-B1a allele headed eight days earlier than those carrying the TaE3V-B1b allele, a significant difference (P < 0.03). These results indicate that under non-vernalized conditions, the developmental process is influenced by a genetic factor at the TaE3V-B1 locus, suggesting that plants carrying the non-functional TaE3V-B1a allele exhibit accelerated development.
To investigate the functional consequences of losing all three TaE3V1 homoeologs, we designed an sgRNA targeting exon 1, located 84 bp downstream from the translation start codon. Using a rapid gene-editing approach, we simultaneously inactivated the three homoeologous TaE3V1 genes to validate their functions. We tested the T2 lines in the same greenhouse under controlled photoperiod and temperature conditions and observed segregation in several visible phenotypes, including variations in flowering time, maturity, number of spikes per plant and plant height within some lines (Figure 5).
In wheat breeding, early maturity is desirable to avoid exposure to the hot and dry summer season, especially in relation to the changing climate. The natural and edited alleles of TaE3V1 identified in this study represent valuable germplasm resources that can be leveraged to accelerate flowering time in wheat cultivars by several days, improving their adaptability to warming climates.

Figure 5. Effects of edited TaE3V1 alleles in transgenic plants.
Photograph showing phenotypic differences among wild-type Bobwhite and seven lines harboring edited TaE3V1 alleles in the T2 families. Three T2 families (B3-4-1, B3-4-4 and B3-4-7) were generated from the T1 plants of B3 and four T2 families (A1-2-1 and A1-2-4 are edited at TaE3V-D1-ED1; A1-2-7 and A1-2-11) were generated from the T1 plants of A1. WT is the wildtype of each TaE3V-A1, TaE3V-B1 and TaE3V-D1 in Bobwhite. ED indicates the editing events that were identified in the T1 plants, and ed indicates the editing events that were newly identified in the T2 plants. * indicates heterozygous. The phenotypic data was the average of 12-24 plants in these T2 families.
| Item | Bobwhite | B3-4-1 | B3-4-4 | B3-4-7 | A1-2-1 | A1-2-4 | A1-2-7 | A1-2-11 |
|---|---|---|---|---|---|---|---|---|
| TaE3V-A1 | WT | WT | ed2* | WT | ed2* | ed2* | ED1 | ED1 |
| TaE3V-B1 | WT | ED1 | ED1 | ED1 | ed2*ed3* | ed2*ed3** | ed2* | Ed2* |
| TaE3V-D1 | WT | ed2* | ed2* | WT | ED1*ed2* | ED1*ed2* | ED1* | ED1 |
| Plant height | 72.1 | 62.1 | 70.52 | 70.2 | 75.8 | 71.1 | 67.8 | 71.8 |
| Maturity index | 24.1 | 46.2 | 80.8 | 44.8 | 59.7 | 63 | 44.2 | 36.9 |
| Spike number | 6.5 | 6.5 | 6.7 | 6.5 | 7.5 | 7 | 6.4 | 6.2 |
Ongoing Testing of Natural Mutants, Tilling Lines and Gene Editing Wheat Plants with Herbicides
We have prepared the following materials for testing plant responses to herbicides:
- Newly generated T0 transgenic wheat plant P16 targeting the VLCFA gene in breeding line OK20708.
- T1 transgenic families derived from the previously generated T0 transgenic wheat plants, H26 targeting the PSB gene in Gallagher and H62 targeting the VLCFA gene in Strad Cl+.
- Two natural cultivars, Mace from the lab and Selbu 2.0 from Washington State University.
- EMS-induced mutants (Table 2).
| Gene | Location | Codon Change | Consequence | Line Name |
|---|---|---|---|---|
| TraesCS4A02G274900 | 4A:584388307 | TGG/TAG | stop gained | Cadenza0905 |
| 4A:584388349 | TGG/TAG | stop gained | Cadenza1710 | |
| 4A:584388425 | CGA/TGA | stop gained | Cadenza1752 | |
| 4A:584388666 | TGG/TGA | stop gained | Cadenza0102 | |
| TraesCS4B02G039000 | 4B:27901981 | TGG/TAG | stop gained | Cadenza1778 |
| TraesCS4D02G036100 | 4D:16147098 | TGG/TGA | stop gained | Cadenza1698 |
