Gene Discovery, Transformation, and Genomic Applications 2023
Gene Discovery, Transformation, and Genomic Applications
Genetic identification of five lines that carry the mutations in all three homoeologous SBEIIa genes.
As planned, we undertook genotypes of breeding lines using KAPS markers for SBEIIa-A on chromosome 2A, SBEIIa-B on chromosome 2B, and SBEIIa-D on chromosome 2D in hexaploid wheat. Among the 258 lines with the PT2190-2196 code number, we found four lines, RT2191-5-1, RT2191-5-4, RT2192-14-3, and RT2192-14-10, each carrying the triple mutants (Figure 1, A-C). We have validated the result using the regular PCR marker method based on the restriction enzyme digestion of PCR products from the same DNA samples (Figure 1, D-F). The SBEIIa triple mutants are expected to have an amylose content of up to 85%, much higher than the SBEIIa double mutants selected in previous years. For unknown reasons, we have struggled to incorporate all three homoeologous mutant genes in a single line for a few years. Finally, we were able to make it happen, but the best SBEIIa mutants will be selected relative to resistant starch potential and field performance. These lines have been planted to reproduce seeds for further phenotyping in the breeding program led by Dr. Brett Carver.
Figures 1A-F.Genotypes of SBEIIa in four breeding lines.
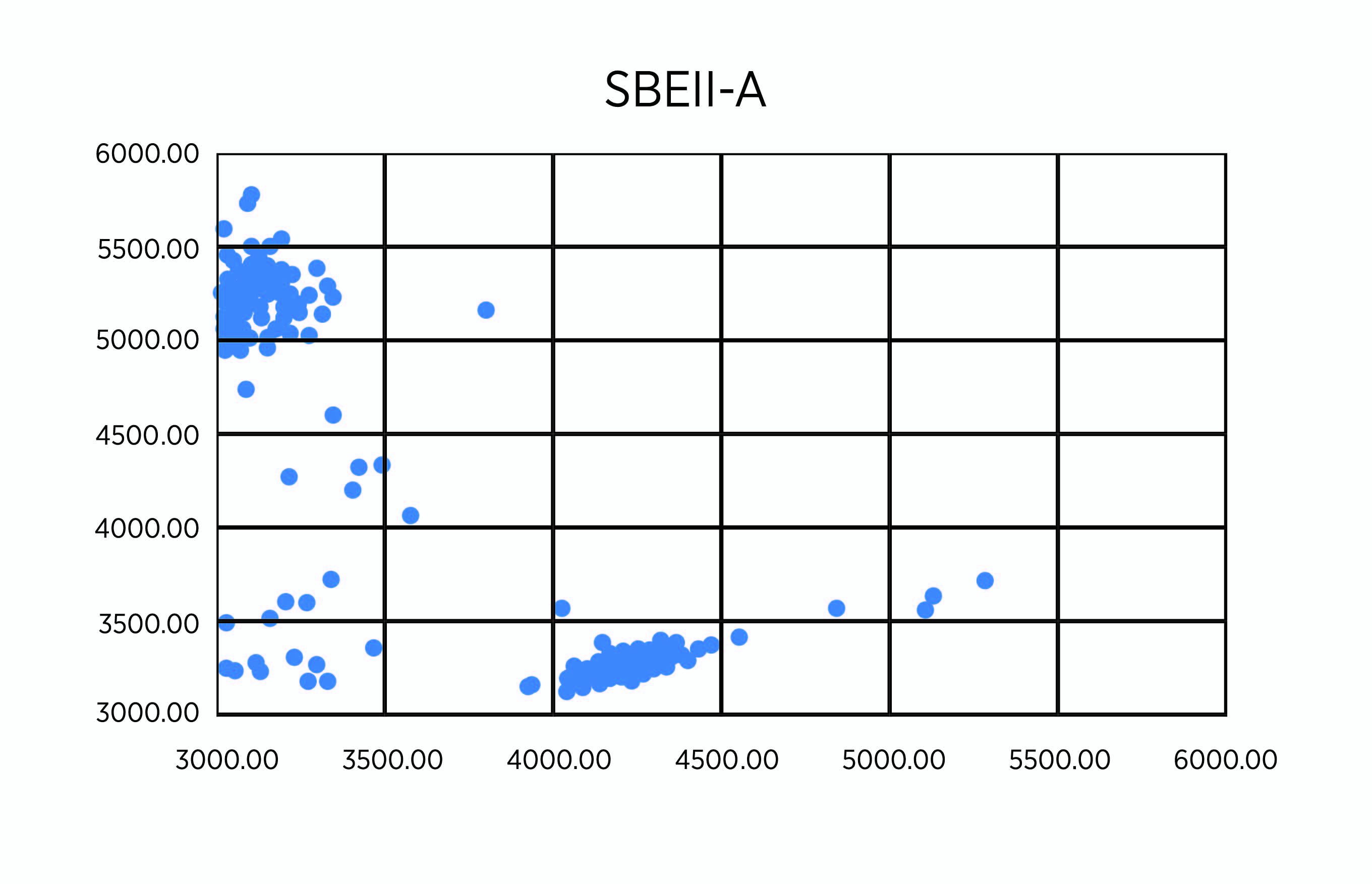
Figure 1A. Genotypes of 258 lines using KASP markers for SBEIIa-A (A).
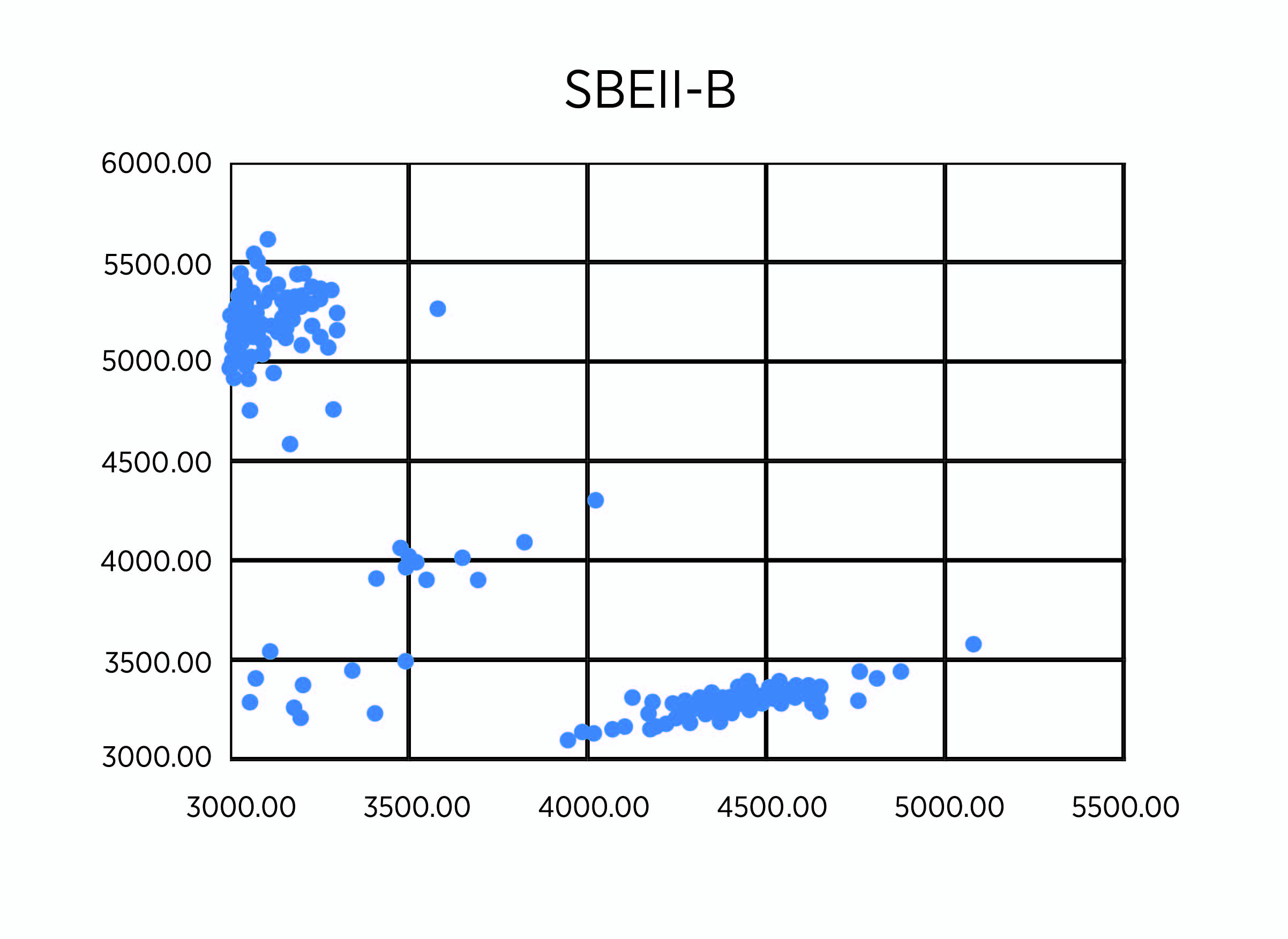
Figure 1B. Genotypes of 258 lines using KASP markers for SBEIIa-B (B).
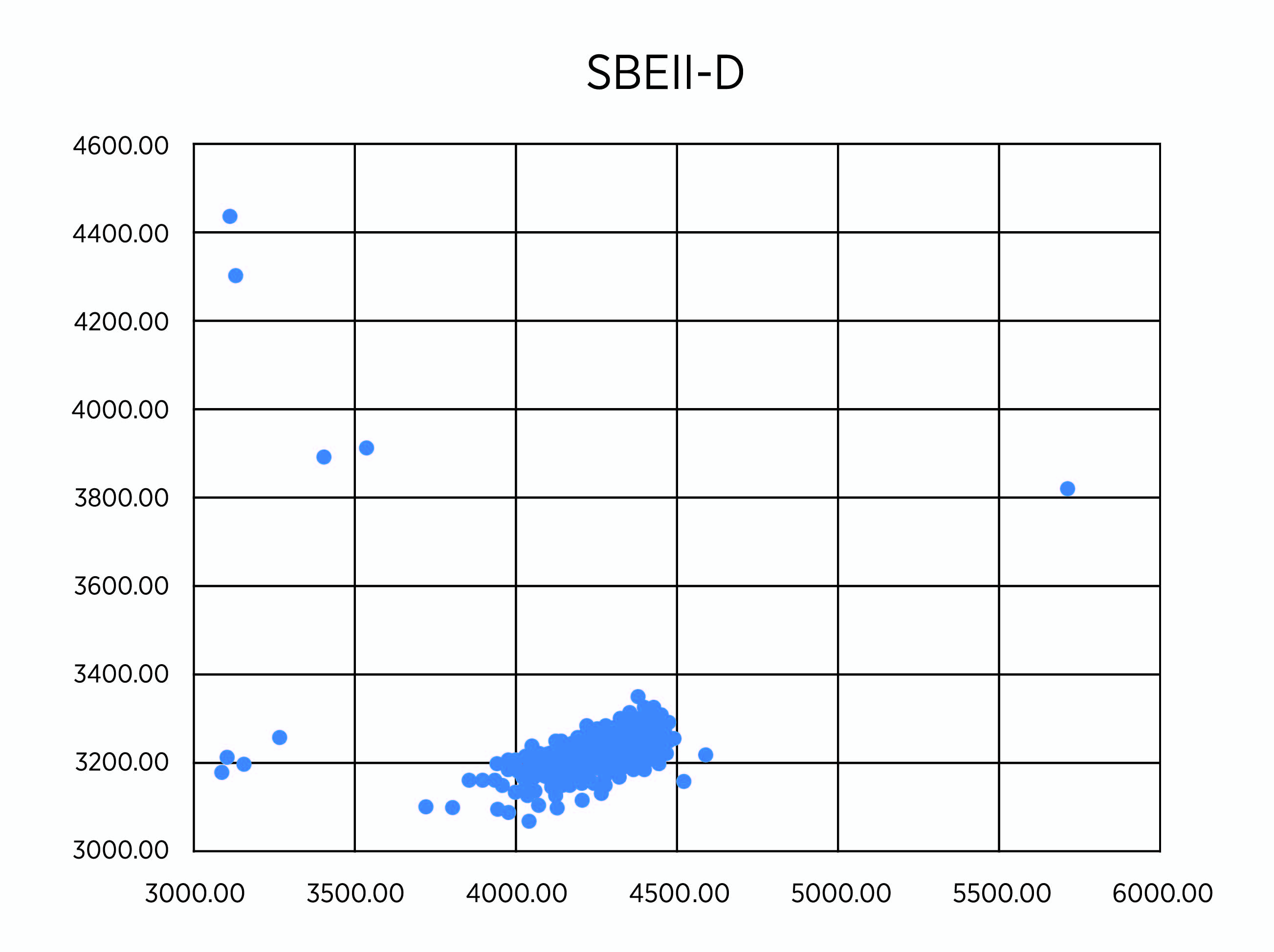
Figure 1C. Genotypes of 258 lines using KASP markers for SBEIIa-D (C).
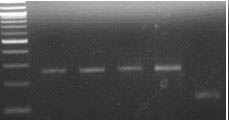
Figure 1D. Genotypes of mutant lines using PCR markers for SBEIIa-A (D).
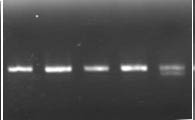
Figure 1E. Genotypes of mutant lines using PCR markers for SBEIIa-B (E).
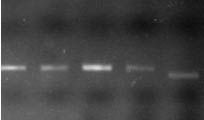
Figure 1F. Genotypes of mutant lines using PCR markers for SBEIIa-D (F).
M indicates DNA markers. 1. RT2191-5-1, 2. RT2191-5-4, 3. RT2192-14-3, 4. RT2192-14-10, 5. RT2190-1-20 (wild-type as a control).
Perfect performance of modified KASP markers for TaAHASL
As planned, we undertook genotypes of breeding lines using KAPS markers for TaAHASL-1B and TaAHASL-1D mutants. Two hundred plants from the lines OK16107133-18HR-4, OK16107133-19HR-3, and OK16107133-19HR-4 were measured in the study. At first, we used a DNA sample pooled from every 20 plants. In the first run with 30 pooled samples, the result showed that all the samples had the wildtype for TaAHASL-1B (Figure 1A). Most of the samples showed the wildtype TaAHASL-1D, but one sample from OK16107133-18HR-4 showed a heterozygous pattern, as indicated with a red cycle (Figure 2B). Next, we extracted from each of the 20 plants constituting the sample with a heterozygous pattern from OK16107133-18HR-4. The result revealed that one of these 20 samples is mutant for TaAHASL-1D (Figure 2C).
Figures 2A-C. Genotypes of TaAHASL in breeding lines.
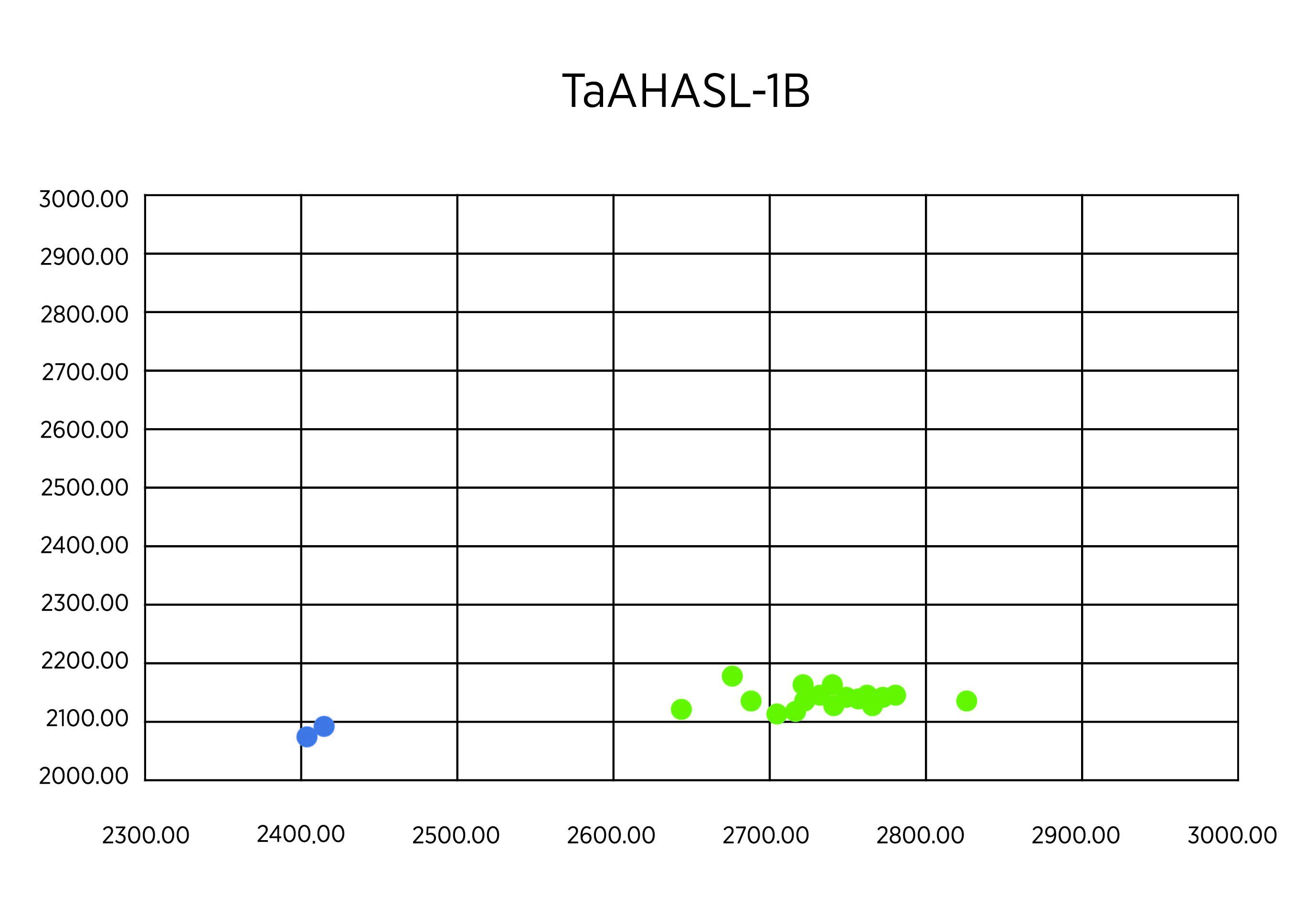
Figure 2A. Allelic discrimination for TaAHASL-1B.
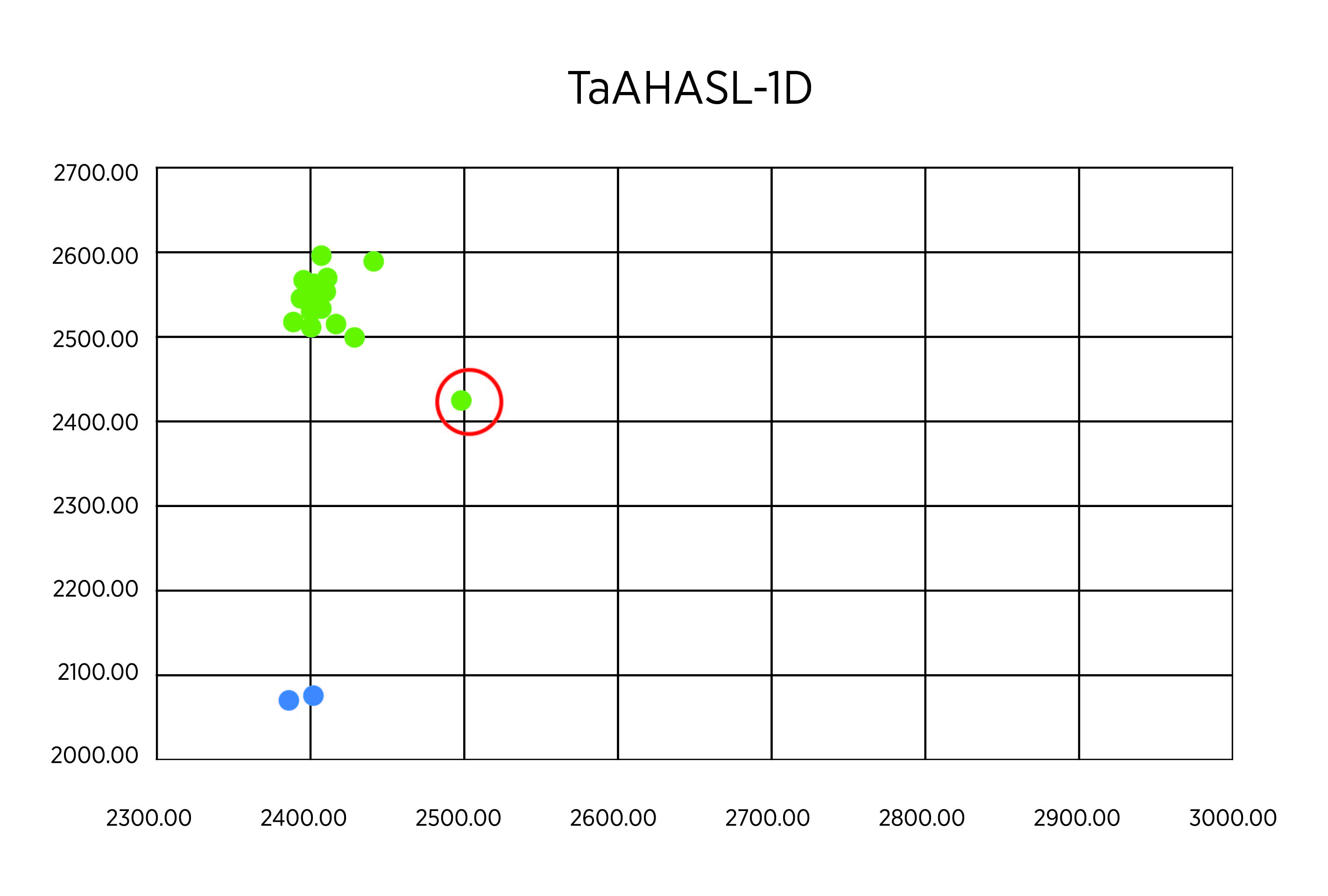
Figure 2B. Allelic discrimination for TaAHASL-1D. The green dots represent the wild-type alleles that are located towards the X-axis. The mutant alleles are predicted to concentrate towards the Y-axis. The blue dots represent water.
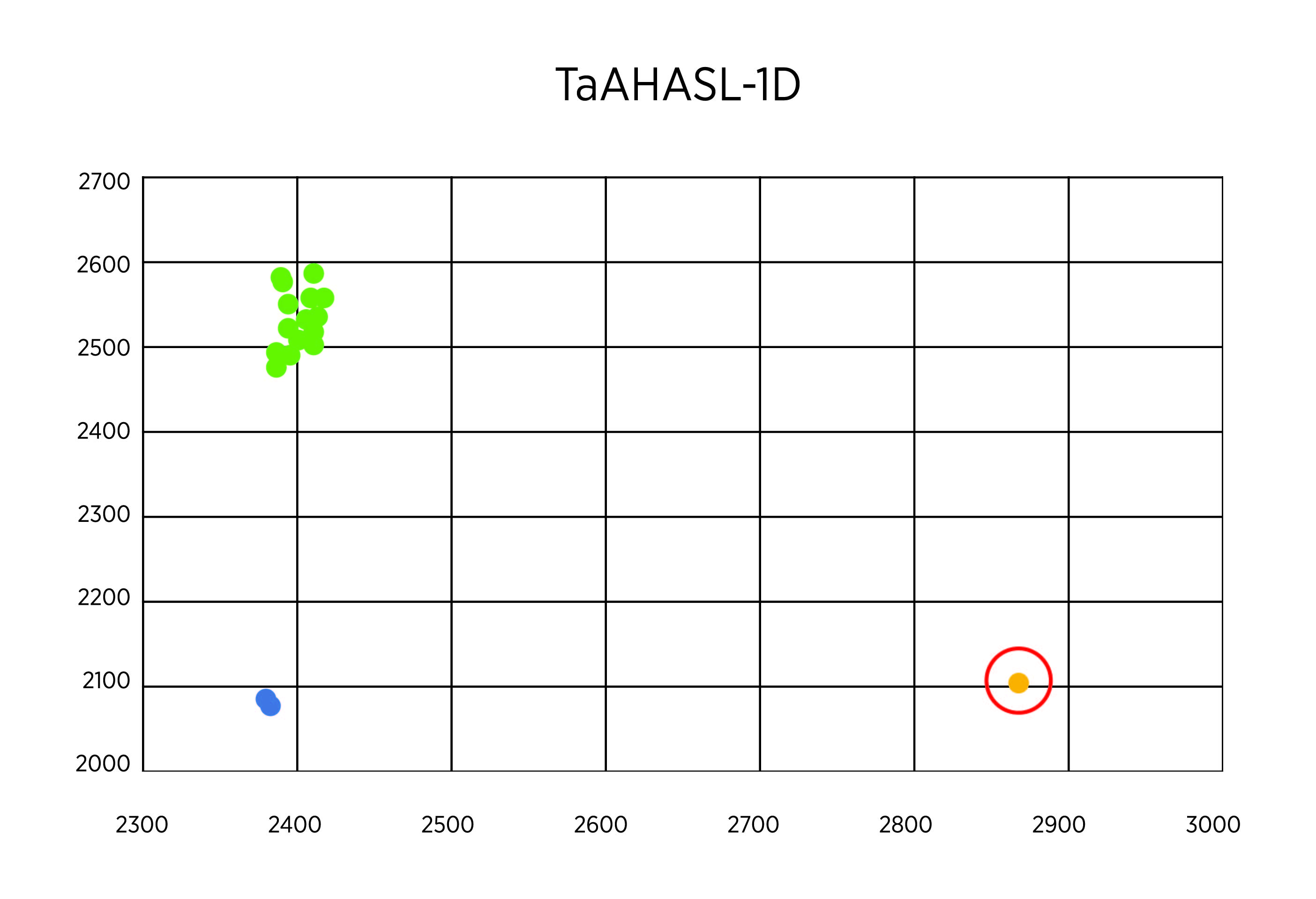
Figure 2C. Allelic discrimination for TaAHASL-1D in 20 samples from OK16107133-18HR-4. (A-C). The green dots represent the wild-type alleles that are located towards the Y-axis. The yellow dot corresponds to a mutant allele located towards the X-axis. The blue dots represent water. (B-C). A red cycle indicates a single mutant DNA that could be discriminated from the total DNA sample pooled from 20 plants.
Establishment of gene-editing platform using locally adapted cultivars
The objective of the present study aimed to establish a gene-editing platform in which a robust, reproducible, and efficient regeneration and transformation system will be used to knock down (or damage) genes for undesirable traits, such as susceptibility to diseases and insects, and knock-in (insert) genes for desirable traits, such as research starch for a healthy dietary fiber. Furthermore, the newly established platform will be workable, regardless of the genotypes, by testing locally adapted cultivars. A successful gene-editing event consists of several critical steps:
- an efficient process of sterilization
- the selection and utilization of culture media with plant growth regulators (PGRs) to be most suitable for the induction of root and shoot starting from embryogenic callus
- the design of a single guide RNA (sgRNA) binding site to targeted genes
- the transformation methods through Agrobacterium or biolistic to transform DNA with gold particles into mature or immature embryos as explants
Spring wheat cultivars Bobwhite and Fielder and winter wheat cultivar Jagger are world-recognized wheat genotypes extensively used in tissue culture and genetic engineering. However, these genotypes are agronomically inferior or have lower adaptations to various environments, lacking the potential of direct deployment when any gene is successfully edited. A reproducible regeneration system with independent genotypes is the essential prerequisite for effective transgenic development in wheat improvement, which is a major bottleneck in the wheat improvement program through gene editing technology.
In our previous study, we modified the protocols of wheat transformation, and we successfully delivered the genes into several local cultivars, including 2174 (Liu et al., Plant Biotechnology, 2018), Billings (Fan et al., Nature Communications, 2021), and Yangmai18 (Zhang et al., Science, 2022). In recent studies, we undertook agronomically superior wheat cultivars with local adaptations to test their regeneration ability. We tested several locally adapted winter wheat cultivars with two regeneration protocols we modified. The winter wheat cultivars included Baker's Ann, Big Country, Billings, Breakthrough, Butler´s Gold, Duster, Gallagher, Jagger, Showdown, Skydance, Strad CL+, Tam112, and Uncharted due to the availability of their embryos. Immature embryos of the cultivars were collected from the field, but they may not be difficult to obtain during the off-season. Results showed that many local cultivars could regenerate on LSZ, CIM, or both media (Figures 3; Figure 4).
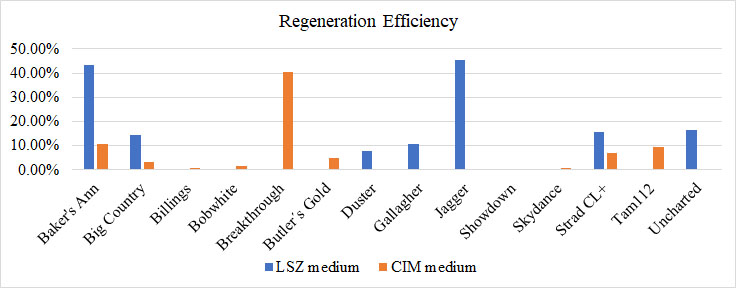
Figure 3.Comparative regeneration ability of winter wheat cultivars on LSZ and CIM media.
Based on the availability of the embryos, Billings, Bobwhite, Breakthrough, Butler´s Gold, and Skydance were tested on LSZ medium only. Duster and Gallagher were tested in the CIM medium only.
It is noteworthy that we have obtained two positive plants, one from Gallegher and the other from Strad CI+, using the constructs with sgRNAs targeting the genes PSB and VLCFA for resistance to herbicide (confidential). Testing to see if the target genes are edited is underway.
In follow-up research, we will use the newly established gene-editing platform to knock down and knock in what genes we want and need. A large DNA fragment or the complete functional gene for the desirable trait could be inserted in any organism's genome using CRISPR-based plant genome engineering, which is selected as one of seven areas of technology by the Nature Journal to watch in 2024. You can imagine how exciting it would be if a desirable gene for elite traits is knocked into our variety without conventional crossing or retaining any foreign DNA from transgenics in the genome of a novel wheat variety in Oklahoma.
Figures 4A-P.Comparative regeneration ability of winter wheat cultivars on LSZ and CIM media.

Figure 4A. Baker's Ann LSZ regeneration.

Figure 4B. Baker's Ann CIM regeneration.
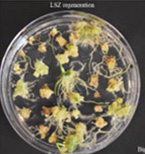
Figure 4C. Big Country LSZ regeneration.
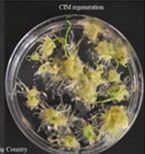
Figure 4D. Big Country CIM regeneration.

Figure 4E. Uncharted LSZ regeneration.

Figure 4F. Uncharted CIM regeneration.
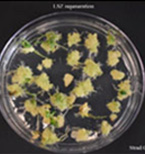
Figure 4G. Strad CL LSZ regeneration.
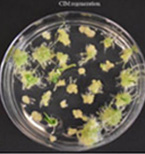
Figure 4H. Strad CL CIM regeneration.

Figure 4I. Showdown CIM regeneration.
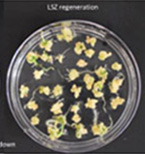
Figure 4J. Showdown LSZ regeneration.
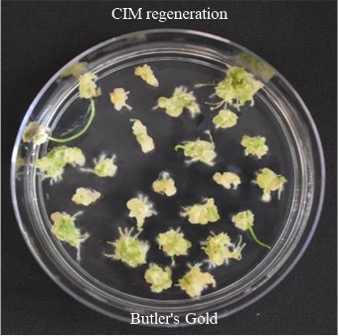
Figure 4K. Butler's Gold CIM regeneration.
Figure 4L. Breakthrough CIM regeneration.
Figure 4M. Skydance CIM regeneration.
Figure 4N. Billings CIM regeneration.

Figure 4O. Duster LSZ regeneration.

Figure 4P. Gallagher LSZ regeneration.
