Wheat Pathology 2021
Development of Disease-Resistant Germplasm
Central to the research direction of the Wheat Improvement Team (WIT) is selecting wheat lines with superior disease resistance. This information is combined with agronomic data to populate all WIT variety development pipelines (VDP). Ideally, the disease evaluation data would be obtained from field trials where the lines are exposed to diseases in a natural setting. However, due to environmental conditions that may not favor disease development, consistent procurement of field data every year is not realistic. Hence, this program is focused on developing and utilizing auxiliary greenhouse tests for the foliar diseases leaf rust, powdery mildew and tan spot.
Testing methodology has been developed to inoculate and evaluate the reaction of both seedlings and adult plants to each of those three diseases. The process involves producing inoculum of each pathogen then inoculating wheat lines in a humidity chamber that critically maintains free moisture on leaf surfaces. Reactions of both seedlings and adult plants are usually evaluated, because wheat can show a susceptible reaction to these diseases (especially leaf rust and powdery mildew) in the seedling stage but then exhibit resistance as adult plants (Figure 2). Such resistance, called adult plant resistance (APR), would be detected in the field when rating in the spring. However, if no disease occurs in a given year, this adult plant type of resistance would be missed. Hence, both seedling and adult plant greenhouse tests are necessary to classify this type of resistance.
During 2020-21, the following seedling and adult plant reactions were determined among several hundred WIT lines in the F7 generation or later. This testing was initiated in September 2020 and concluded in April 2021. It is anticipated these evaluations will likely increase as they enable line advancement in the VDP. Currently, only under rare circumstances would a leaf rust susceptible line be advanced. Moderate susceptibility to powdery mildew or tan spot in greenhouse tests may be allowed but not if coupled with highly susceptible reactions in the field (if available). Released varieties by WIT have progressed toward lesser dependence on requisite fungicide applications. Testimonial to this practice is the recent releases of Skydance, Uncharted, Strad CL+, Butler’s Gold and Big Country.
| Leaf Rust | Powdery Mildew | Tan Spot | |
|---|---|---|---|
| Seedling | 7,100 | 5,500 | 10,800 |
| Adult Plant | 7,100 | 5,500 | - |
Evaluation of WIT lines was also conducted in 2020-21 field nurseries. Seven hundred WIT lines were planted in a field nursery to test for reaction to the virus disease complex known as wheat soil-borne mosaic/wheat spindle streak mosaic (WSBM/WSSM). Although disease symptoms were not as strong as in previous years, 2021 ratings identified nearly 75 lines as susceptible to the WSBM/WSSM complex. In the future, combining observations from this field nursery with marker assisted selection (MAS) will help to ensure that OSU-released varieties intended for the central corridor will have resistance to the WSBM/WSSM complex.
Another field nursery was used to evaluate the reaction of 240 advanced lines to barley yellow dwarf and stripe rust. In 2021, both diseases were sufficiently severe in this nursery to allow rating of these lines to both diseases (Figure 1). This information contributed directly to the selection of elite lines with a broad disease resistance package (See Table 2 in Carver’s report). Altogether, 23,000 plant ratings were generated from greenhouse testing and more than 1,500 ratings from OSU field nurseries.

Figure 1. Presence of severe stripe rust on a susceptible “spreader” strip in the stripe rust/barley yellow dwarf field nursery in April 2021.
Evaluation of other diseases was conducted through collaborations with pathologists in other states. For example, evaluation of WIT lines for reaction to stripe rust was conducted with collaborators in Kansas and in the Pacific Northwest, and evaluation for reaction to wheat streak mosaic was conducted with a collaborator in Nebraska. These external evaluations in combination with OSU greenhouse and field testing, breeder trials and extension variety trials provide the engine for driving comprehensive disease resistance in OSU varieties.
Tan Spot Pathosystem
Tan spot of wheat, which is caused by the fungus Pyrenophora tritici-repentis (PTR), can significantly reduce yield, especially in no-till production systems where wheat residue remains on the soil surface. This residue harbors PTR inoculum that infects the new wheat crop.
The pathogenicity of PTR mainly depends upon the production of several host-selective toxins (HSTs) – including PtrToxA, PtrToxB and PtrToxC – with PtrToxA being the most common HST. Based on the production of HSTs in five wheat differentials, eight races of the pathogen have been recognized, known as races 1 to 8. Phenotyping of 40 PTR isolates from Oklahoma on five wheat differentials showed that 36 (90%) isolates correspond to race 1, and four (10%) isolates correspond to race 3 (Figure 2).
Spray-Induced Gene Silencing (SIGS) was used to attempt gene silencing in PTR. However, first attempts to silence the phytoene desaturase (PDS) gene of wheat were not successful as indicated by the lack of photobleaching of leaves in the differentials Glenlea and TAM 105 after seven days post infiltration (Figure 3). Hence, this method is not suitable to silence the Tox A gene of PTR. Future studies will focus on examining the Virus-Induced Gene Silencing (VIGS) method targeting the PDS and ToxA gene of PTR. Ultimately, since PtrToxA is a major pathogenicity factor, the ToxA gene was cloned into the E.coli SHuffle expression system, and the protein will be purified and tested on wheat germplasm for an improved and faster identification of tan spot resistant cultivars.
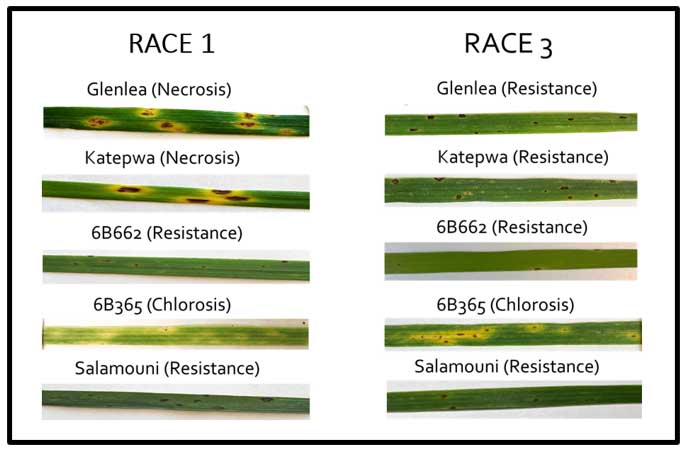
Figure 2. Race characterization of PTR isolates based on symptoms produced on five wheat differentials.
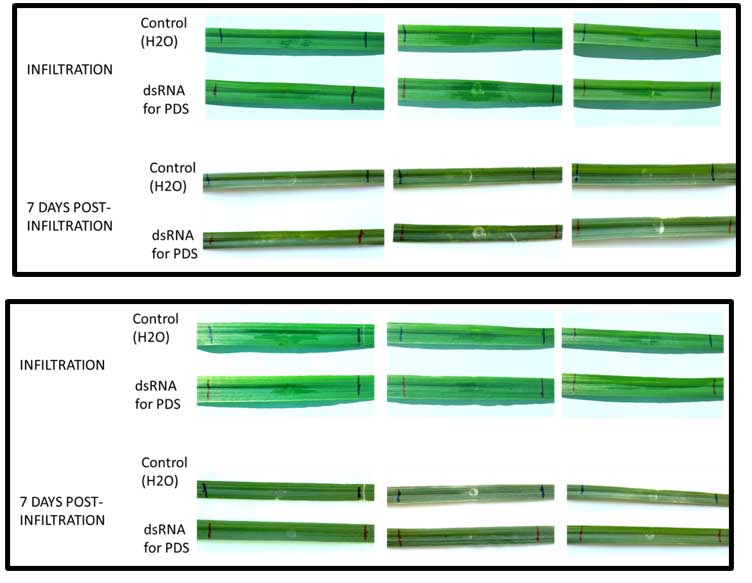
Figure 3. Infiltration of dsRNAs targeting the PDS gene and water (control) on wheat cultivar Glenlea (top) and TAM 105 (bottom) at 7 days post-infiltration. Note the lack of any photobleaching that would indicate successful gene silencing.
